Real Time measurement of nanosized magnetite synthesis
With a custom made magnetic susceptometer and a custom made mixing unit, we can follow – at a well below sub second pace – the actual chemical reaction between an iron salt solution and a base where superparamagnetic nanosized magnetite particles are synthesized. A major finding is that the reaction with ammonia is orders of magnitude faster than with sodium hydroxide, although ammonia is a much weaker base. A puzzling observation nobody has volunteered to explain.
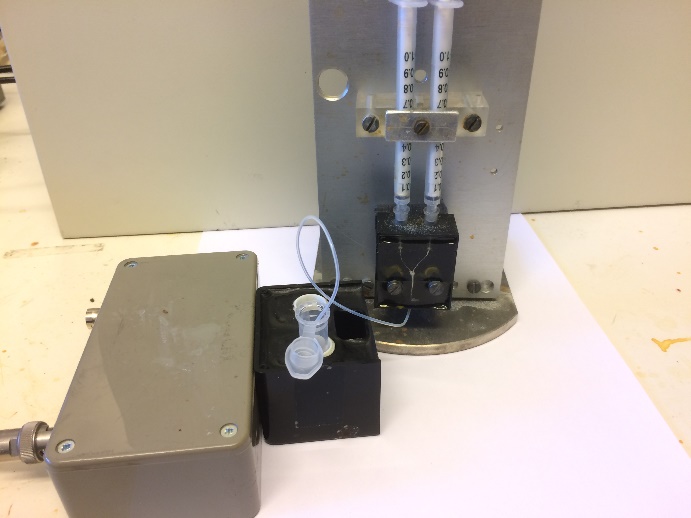
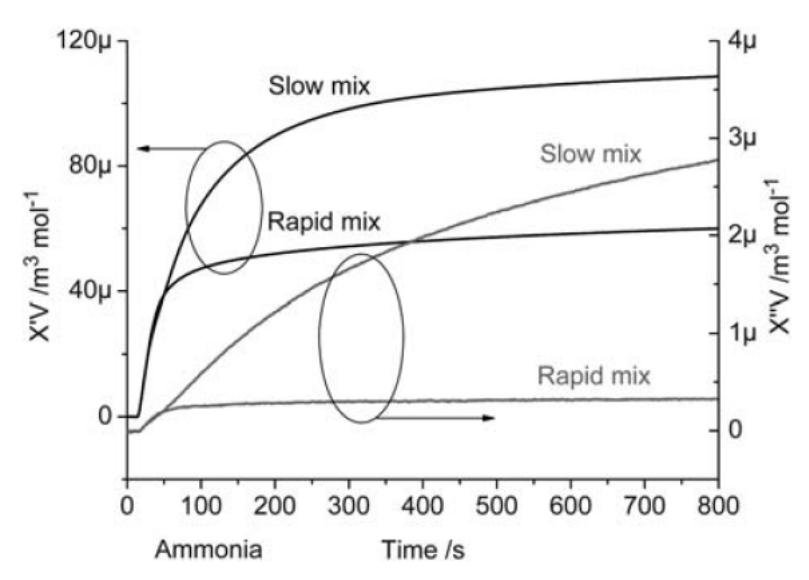
Collaboration with Richard Olsson, Fiber & Polymer Dept., KTH
V. Ström, R. T. Olsson och K. V. Rao, "Real-time monitoring of the evolution of magnetism during precipitation of superparamagnetic nanoparticles for bioscience applications," Journal of Materials Chemistry, vol. 20, no. 20, s. 4168-4175, 2010
